Cybersecurity and connectivity to monitor oral anticoagulation therapies
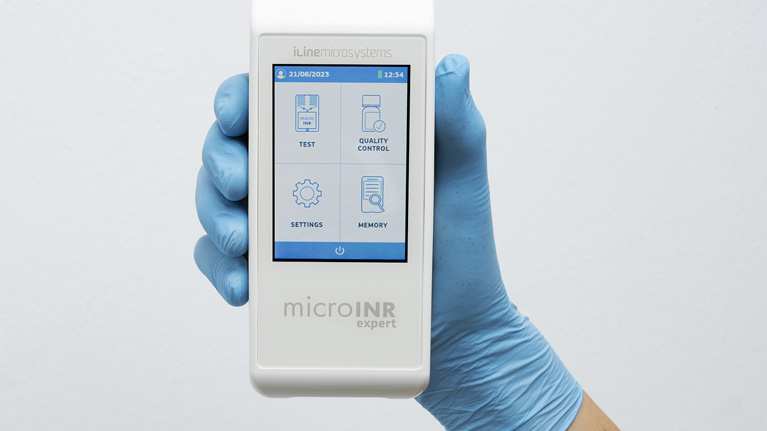
Tekniker technology has applied its state-of-the-art technology and expertise to develop the professional microINR Expert coagulation meter. One of the most outstanding features of this products is its full data traceability.
In an increasingly digitalised healthcare environment, new connectivity and cybersecurity technologies are nowadays essential to ensure correct patient data use and traceability.
Tekniker, a technology centre with extensive experience in the area of electronics and the design of precision medical devices, has collaborated in the fine-tuning of a new generation of in-vitro diagnostic systems made by iLine Microsystems.
More specifically, a team of Tekniker professionals has been involved in the area of mechanical and hardware design as well as in the implementation of the embedded and cybersecure software used by the innovative portable microINR Expert coagulation meter.
MicroINR Expert will now join the list of products developed for iLine Microsystems. The most salient feature of this coagulation meter is that it has been designed to only be used by healthcare professionals. Thanks to the communication protocols set up for the different connectivity options, it is easy to integrate the device in different kinds of hospital management IT systems.
Jorge Berzosa, a Tekniker researcher, explains that “by working together, iLine and Tekniker have developed a lightweight, compact, cybersecure and connected device fitted with a high-resolution touch screen and an improved user interface designed to meet usability standards. The device, moreover, allows hospital data compiled in data bases to be sent directly and automatically via wifi, Bluetooth LE 5.0 or Ethernet”.
In order to guarantee that stringent security and traceability standards are met, the system incorporates a bar code that identifies patients and healthcare professionals.
Official launch in the European market
Subsequent to obtaining the CE marking, the microINR Expert device was launched in the market in November 2022 and was widely acclaimed by iLine Microsystems’ most important customers.
In order to do so, Tekniker’s know how was essential to develop embedded medical devices and meet specific sectoral regulations (ISO 13485, IEC 62304, ISO 14971), as well as usability and security standards (IEC 60601, IEC 62366, UL2900).
